BIR presents MRI safety initiatives

The British Institute of Radiology (BIR) has multiple specialist interest groups (SIG), each with a multidisciplinary membership. We are very grateful for the work of each group, which underpins our effectiveness. BIR members are encouraged to join and contribute to our SIGs. Each group helps develop and deliver educational events and webinars as well as contributing to national procedures and processes, working alongside our partners.
Below are some of the recent initiatives to which members of our MRI SIG have contributed:
IPEM MRSE certificate of competence
All clinical MRI units are advised to appoint an MRI safety expert (MRSE) to support and advise on matters of MRI safety. To help ensure that individuals taking on the role of MRSE have appropriate knowledge and experience, the BIR and other professional bodies contributed to the development of the Institute of Physics and Engineering in Medicine (IPEM) MRSE Certificate of Competence scheme.(1) This scheme requires candidates to demonstrate sufficient knowledge by passing the MRSE examination provided by the American Board of MR Safety (ABMRS).(2) Members from the BIR have contributed to developing international versions of the ABMRS examinations as well as contributing to the move to electronically administered examinations, which mean candidates can now book to sit an examination on a day that is convenient to them at one of several test centres across the UK. An additional requirement to provide a portfolio of examples to demonstrate sufficient experience is assessed by an IPEM MRSE panel.
Generic implant safety procedures
Another recent MRI safety initiative has been the development of generic implant safety procedures (GISP) that aim to define a process for managing patients with certain types of implants where the MRI safety risks are low. Th is incorporates scope for an evidence-based risk-benefit decision to scan some groups of patients with certain types of medical implants under locally approved conditions, without seeking to identify the exact make and model of the implant and subsequent assurance of MRI safety from the implant manufacturer. A multi-professional group(3) has developed guidance on a framework for developing GISPs(4) and is continuing to work towards generating a set of nationally agreed GISPs.
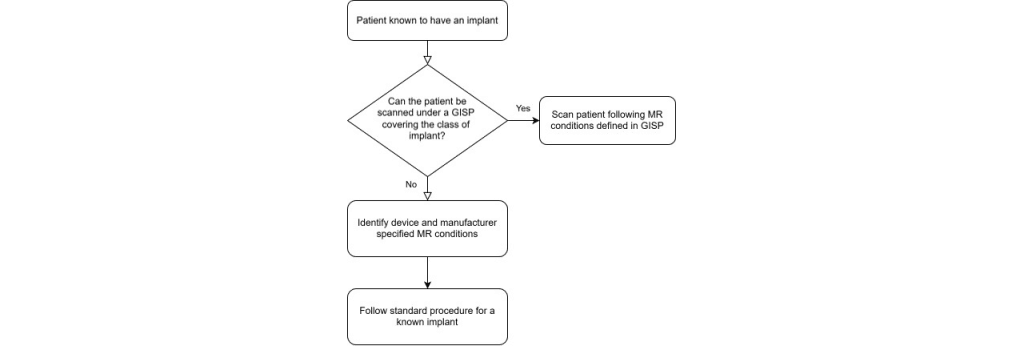
The GISP process occurs before the normal procedure of rigorous device identification and determination of manufacturer provided MRI conditions. In appropriate cases, this can avoid delays associated with the labelled procedure.
MRI safety e-learning
Finally, the e-learning MRI safety programme developed by the BIR and other professional bodies continues to go from strength to strength. Hosted on the elearning for healthcare platform, this is freely available to anyone working in the NHS or at a UK academic institution via a number of routes(5-7) and to others via the eIntegrity platform.(8)
Lead picture: Geoff Charles-Edwards, Mark Radon, BIR Magnetic Resonance Special Interest Group (SIG) and BIR President Nick Screaton.
References
- 1, www.ipem.ac.uk/your-career/professional-registration/mrse-certificate-of-competence
- 2, https://abmrs.org
- 3, www.ipem.ac.uk/resources/mri/generic-implant-safety-procedures-gisps-task-and-finish-group
- 4, Ashmore et al. Br J Radiol 2025;98(1167):336-44. https://pubmed.ncbi.nlm.nih.gov/39535863
- 5, Direct access via elfh website. www.e-lfh.org.uk/programmes/mri-safety
- 6, Via NHS electronic staff record. https://my.esr.nhs.uk
- 7, AICC links from organisations’ own learning management systems, https://portal.e-lfh.org.uk/home/aiccreport
- 8, eIntegrity platform. www.eintegrity.org/healthcare-course/mri-safety


